Sicherheit Medizintechnik Sicherheit als Maxime
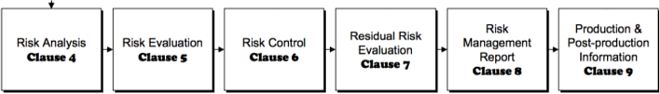
Risk management according to DIN EN ISO 14971
The safety of patients, doctors and nurses is of great importance to Interelectronix .
By introducing a risk management system for medical devices in accordance with DIN EN ISO 14971, we are more than living up to our maxim.
Regardless of the legal regulations, the application of risk management from the product idea to the market launch is consistently practiced at Interelectronix across all processes and departments and includes the following components:
-Risk analysis
-Risk assessment
- Risk control
- Analysis of risk-relevant information from market observation
- Evaluation of lessons learned from the risk management process
An important element of our risk management is a periodic reassessment of all measures after they have been implemented. However, this does not end with the delivery of a product, but specifically includes the market observation of the product over its life cycle phases.
If necessary, a dynamic adjustment of the risk management for medical devices is carried out through close observation of the products and their functions in use, taking into account the respective hazard types.
Medical technology products must be both safe and efficient to use. Consequently, it is only logical to apply risk management already in the development phase.
The primary goal of Interelectronix risk management is therefore to ensure the elimination or minimization of risks through conceptual or constructive measures.
This starts with the design of a product and extends to all processes, procedures and production procedures.
Risk analysis is an important part of risk management
The performance of a risk analysis is required by the approval procedures in the EU and by the FDA. It is an efficient way to direct product development and validation efforts to where the greatest risks can arise.
The Failure Mode and Effects Analysis (FMEA) has so far been an essential component of quality assurance before the start of series production. However, risk analyses according to DIN EN ISO 14971 differ considerably from FMEA.
The failure mode and effects analysis is therefore no longer recognized by the approval bodies for the approval of devices according to EN 60601-1 3rd Edition.
MOPP - Means of Patient Protection
The IEC 60601-1 standard defines "Means of Patient Protection (MOPP) to reduce the risk of electric shock to the patient".
As a result, medical devices must always be equipped with two independent protective measures for patient protection in order to be "first fault-proof".
If one of two protective measures fails, the safety of the patient must not be compromised. Depending on the type of hazard, several protection systems usually work side by side, which are intended to counter different hazard potentials.
-Dielectric strength
A high dielectric strength is intended to protect against the pulsed overvoltages that occur frequently in electrical systems.
Application-specific insulation structure
An application-specific insulation structure is intended to prevent the risk of inadequate insulation due to manufacturing defects or the aging process.Clearance and creepage distances
Damp or dusty environments can lead to electrical flashovers, which should be avoided by means of clearance and creepage distances.Leakage currents - leakage currents
Patients should be protected from acting leakage currents by reducing them to the maximum values of the leakage currents (standard depends on the device).Protective conductor connections
Protective conductor connections are designed to dissipate hazardous currents so that they do not reach the patient.Patient leakage current (DIN 60479-1)
The patient leakage current is created by a faultless electrical circuit in the device, which flows either directly via the protective conductor or indirectly as a device leakage current via conductive device parts to the ground, thus posing a danger to patients.
Touch systems and HMI according to IEC/UL 60601-1 standard
Especially in medical technology, device safety, personal protection, high hygiene, long service life, reliability and efficient functional integration are of utmost importance. All touch panels and touch systems developed by Interelectronix are subject to the high safety requirements for medical devices in accordance with the basic standard IEC/UL 60601-1.
The IEC/UL 60601-1 standard defines general requirements for the basic safety and essential performance characteristics of medical electrical systems connected to a supply network that are intended for the diagnosis, treatment or monitoring of a patient. The European standard EN 60601-1 is identical to the IEC/UL 60601-1 standard.
Due to their high reliability and advanced technology, their innovative product design and intuitive usability, Interlecronix touch systems are used by
- Ultrasonic devices
- X-ray machines
- Computed tomography scanners
- Laboratory analysis equipment
as well as
- in the operating room
- in dental medicine
- in patient monitoring
- and patient registration
used.
In the case of touchscreens used in medical technology, the patient leakage current can be avoided either by a specific design, insulation or the use of suitable materials such as plastic housings or front panels.
Every touch panel used in medical technology is subject to a precise check of the exact current flow to detect and avoid device leakage current.
Protection tests according to IPX1 to IPX8
Touchscreens in medical devices are subject to particularly high safety requirements. For example, the touch systems often have to work error-free for years under difficult conditions and at the same time ensure full protection for patients and operating personnel.
Touchscreens used in medical technology are subject to considerable exposure to cleaning agents, disinfectants, water, vapors, acids or body fluids. In addition, the ingress of foreign bodies and dust, as well as contamination with bacteria and viruses, must be virtually eliminated.
According to the respective requirements and areas of application, Interelectronix develops ready-to-install touch systems that comply with the various protection classes and standards from IPX1 to IPX8:
- Protection against foreign bodies (DIN 40 050 Part 9 or DIN EN 60529)
- Protection against water (DIN 40 050 Part 9 or DIN EN 60529, water protection class tests)
- Shock resistance (DIN EN 62262)
As a specialist for high-quality and ready-to-install touch systems, Interelectronix has many years of experience in the development of touchscreens, touch panels and complete touch systems that meet the special requirements of medical technology for safety, durability and patient protection.
A wide range of glasses, housing materials, high-quality seals and lamination techniques enable the construction of IPX1 to IPX8 compliant HMI (Human Machine Interface).